Volatile
Volatile liquid confirmed xD
As always, if you like the comic, please consider donating on my patreon. Even one dollar goes a long way.
Don’t forget that I stream every Sunday! You are more than welcome to join in on the fun.
The stream is hold at the following hour:
UTC: 1PM
EDT: 9AM
PDT: 6AM
CEST: 3PM
Don’t forget to vote for Twin Dragons at Topwebcomics!
http://topwebcomics.com/vote/21879
Thanks for voting!
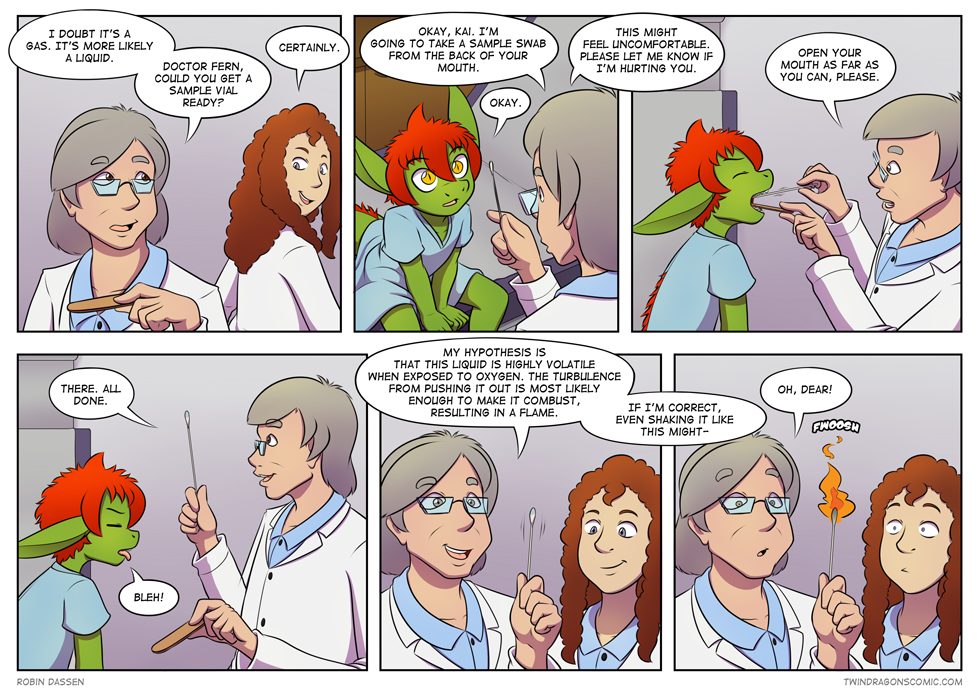









Well shucks, now the docs have to get another sample! Kaya’s turn?
but they need a sample of both, so Kai has to go again as well xD
The Twins won´t get declared weapons of war or something like that now, i hope?
After all they are walking, super strong flamethrowers. With a sense of humor.
Oh, any chemistry experts here? What’s volatile enough to auto combust on contact with oxygen and might reasonably be found in a living organism?
Phosphorus might fit the bill
(although it’s been a while since I’ve taken Biology or Chemistry)
or could be something sodium based. which might be easier to ‘recharge’ given that sodium is fairly common in modern foodstuffs, due to the high amounts of salt being used as a preservative and favoring agent.
There are a few hypergolic compounds that will auto-combust in exposure to oxygen, usually in the presence of a catalyst. For example hydrazine, in contact with oxygen and an iron catalyst will burn. That is what is used on many spacecraft to power manoeuvring thrusters.
Needs to be nontoxic and stable. Hydrazine is very toxic. Needs to not need rare ingredients. Hypergolic with air, or there might be two chemicals — two nozzles. One could be the fuel, the other mostly a trigger, although it seems to catch spontaneously well enough.
It doesn’t necessarily have to be nontoxic. As an extreme example, snake venom is very toxic, yet is (obviously) produced and used by snakes.
Correct me if I am in any way wrong, but the substance is most likely sodium tert-butoxide (NaOC(CH3)3) or potassium tert-butoxide (POCH3(CH3)3), dissolved in a solvent such as diethyl ether (C4H10O), tetrahydrofuran (C4H8O), benzene (C6H6), Toluene (C7H8) or hexane (C6H14). sodium- and potassium-tert-butoxides are solids, but can be dissolved in the solvents making it a liquid. Also, the tert-butoxides react with moisture in the air, releasing hydrogen gas maybe fast enough to heat up and combust. This hypothesis makes most sense because both substances (in the comic and irl) are liquids, both react to turbulance in the air, and the irl substances are made up of Hydrogen, Carbon, Potassium/Sodium, and maybe oxygen, all very common in food. That is my theory.
I found a Wiki article if we assume a one part fuel — https://en.wikipedia.org/wiki/Pyrophoricity
This lists several possibilities, including the Pern option. None of them are plain CHON materials. P2H4 – the option used on Pern sounds the least poisonous to me. Most of the others involve elements that aren’t normally eaten, eg Boron, Lithium,m Zinc, Aluminum, etc.
Plus, the flame is shown to be orange. This rules some of them right out. Triethylborane for example is green when it burns. And requires you to eat boron. Surprised they didn’t use that on Pern, the fire lizards and Dragons are all highly tolerant of Boron, which is not true for earth life.
Phosphine can get toxic as all hell. Actually the firestone on Pern contains P2H4, which is a bit more stable but sublimates at room temperature. The high-grade ore has plenty of it trapped in the rock matrix. Dragons macerate the ore and swallow it down into the fire-stomach which is adherent to the food-stomach. Basically, the same molecular mechanisms which generate acid for the food stomach make the fire-stomach very alkaline, which, with some hardy enzymes and some iodine, convert the P2H4 to PH3 (phosphine). That gets belched up and…fwoosh.
Though phosphine is pretty toxic…in the first novel, it was referred anecdotally that one dragon died of burns and phosphine poisoning.
Boron isn’t too bad if it’s bound up in the right molecular structure, and it depends on the dose.
I didn’t remember the bit about a dragon dying of the stuff. P2H4 was used by Nasa to start rockets, also used to start SR71 engines. The fuel it used is extremely hard to ignite.
Still, it uses too much Phosphorus to be reasonable.
PS – Pernese dragons are totally immune to Boron. The planet is loaded with it.
They referenced it early on in “Dragonflight” and “The Masterharper of Pern.”
Noooo! Now we’ll never know what it is!
unless we take another sample :p
So he’s got flammable spit. Better keep him away from fireworks and other explosives then.
It’s not their spit/saliva. The doctor specifically mentioned in the last page that they have two valves in the back of their mouths that the flammable liquid comes out of.
Really? I just assumed that liquid was Kai’s saliva considering that’s what doctors use that cotton swab for.
His parents know.
https://www.twindragonscomic.com/comic/happy-new-year/
Don’t worry, his spit isn’t flammable by default :p
It’s what comes out of the tiny valves at the back that is xD
They’re going to need a new sample
because of your pyro skills.
They’re working out how you work
and matching action with wills.
The Doctor’s on his own,
even with the other there;
she’s got to be concerned
for the effects upon her hair.
Maya’s waiting her turn
beside her bro on the bed
and, with enthusiasm,
she’ll do anything that’s said.
Maya? I wrote this on my tablet. Spell-check got me.
I don’t care this is amazing. Keep it up.
https://en.wikipedia.org/wiki/Pyrophoricity
One sure thing : the check-up is becoming a total anatomical study , now the doctors have finally succeed to “grab” two mythical hybrids . I hope Marco has a lot of free time today ?
He’s not making it out of the hospital anytime soon xD
Interesting, so it’s the “ignites on contact with oxygen” method but it still needs a moderately violent shock to set it off and doesn’t just essentially explode as soon as oxygen touches it. That helps fix the problem of how that liquid somehow getting accidental exposure to oxygen could be hazardous for the twins if it was still in/on them somehow when it came in contact with oxygen.
Yeah, the body has a tendency of making things relatively safe for yourself ^^
FOR SCIENCE!!!
Dragon science! xD
on the pro side: my hypothesis is proven, on the con side: how the heck are we going to analyse something *that* volatile?!
very carefully
Take the sample and slowly put it in a test tube filled with CO2 or water. As he said, it needs O2 so no O2 = no fwoosh. That is assuming it doesn’t create it’s own oxygen. 🙂
It might even react with water or CO2, so fill the tube with helium.
Reminds me this one: Here’s a list of things that make Azidoazide Azide explode:
moving it
touching it
dispersing it in solution
leaving it undisturbed on a glass plate
exposing it to bright light
exposing it x-rays
putting it in a spectrometer
turning on the spectrometer
ABSOLUTELY NOTHING. It was just sitting in dark room and suddenly it exploded. Probably someone said something bad about it.
… compared to that, this is safe to analyze.
Found the source: https://nerdfighteria.info/v/ckSoDW2-wrc/
Reminds me of this article:
https://blogs.sciencemag.org/pipeline/archives/2008/02/26/sand_wont_save_you_this_time
Warming it up to minus oh my god that’s cold will do it as well.
To take a sample safely, I’d suggest sealed syringes (filled with an inert, non-oxygenated, cohesive fluid if necessary) and slowly draw some from each opening (separately). After that it’s a matter of proper handling.
By being way more careful xD
So they basically Gleek volatile fluid from a gland at the back of their throat. That’s awesome. 🙂
Yeah, it’s something like that :p
“Volatile. Heheh! Like Kaya?”
“What? Oh, nonono; wrong definition of ‘volatile’!”
Alternative version:
“Volatile. Heheh! Like Kaya?”
*SMACK*
“Ow?”
Haha, be careful around Kaya xD
The “turbulence” idea is basically compression. On an atomic or molecular level, when a liquid is pushed through air, it compresses against other air molecules. So their fire breath comes from something super-sensitive to compression.
I know there’s a stereotype in cartoons about nitroglycerin being volatile to the point that spitting it would cause an explosive reaction. As I understand it, that’s actually true. Maybe that’s the liquid? I’m kinda surprised that they don’t naturally burn their tongues.
Nitroglycerin detonates rather than explodes. It would do so in the bladder at that temperature. It IS refreshingly free of funny elements.
Yeah, that might be right. I kinda see it as it being vaporized when they push it out as well. So they don’t shoot out a beam of liquid, but more like a spray bottle. The resulting ‘fog’ (there’s probably a better word for it in english, but I don’t know it) would easily ignite in an oxygen-rich environment.
The word you’re looking for is aresol or to aresolize. relatively safe in fluid form, but mix the vapor into air and it becomes volitile. It’s what fuel injectors do in a gasoline engine.
@Cliff Are you sure you don’t mean “atomize”? At least, that’s what happened in a carburetor. It’s the process in which a large amount of air is forced across a tiny amount of liquid, and done so inside a conically-shaped container. It causes somewhere around 80%-90% of the gasoline to be vaporized.
You got me there. I wasn’t aware atomize had a definition other than “to be split into atoms.” aresol was the closest I knew to it. Good on ya
Good example, for a powder. Coffee creamer. Sitting in a little pile, it is difficult to light, harder to make it stay lit.
Blow it into a cloud, foom. Flour much the same.
I think he just realized how much of an oopsie he made. The twins are going to have such an amusing story to tell their friends regarding their check-ups.
And now I’m imagining Cleo coming in with gourmet popcorn to eat while Kaya regales her with the story of what happened at her check-up, I don’t know why but that just screamed Cleo to me, made all the more hilarious by Cleo asking Kaya to cook her popcorn before she is told the story about what happened at the hospital. Kaya is totally the kind of person to share what happened at the hospital with Cleo.
That all said, I hope we get to see Cleo’s dad, and his possible interaction with Kaya, soon.
Gourmet popcorn xD
Yup, she could definitely pull that off and no one would question it xD
Congrats on making your first DIY match XD. Also i love the sudden expression change in panel 5 to 6 from observes face to oh no
They realized just how volatile this thing is xD
There are many common tropes behind dragon fire science. Generally it either boils down to magic or explosive gas. Very rarely is a pressurized liquid ever considered, even though there are some select materials which in theory could make such a reaction possible. Regardless, I suppose I shouldn’t be surprised at this point that once again Robin has taken a different spin on something that can normally be predicted. It’s blatantly apparent that Robin is a creative genius, and has plenty of experience doing what they love. Keep up the good work my friend. Artists like you are the reason people try to find webcomics in the first place. You’re truly a pro. As always, stay awesome!
Oh, and Happy New Year everyone! I’ve Been so busy with all the college applications I left until the last week to do that I forgot what day it was! Robin’s card reminded me. Best of luck to all of you.?
Thanks, Alpha! 😀
I took inspiration from the bombardier beetle. That insect can shoot a burning liquid as a defense mechanism. Easy to see how I got from there to this xD
Happy New Year, Alpha 😀
Just a spoonful of sugar
and the fire comes out
XD
In a most igniteful way.
chim chimney, chim chimney, chim chim cher-ee,
the doc is as lucky, as lucky can be
xD
Super-Cali’s burning down; the trees are quite combusted?
It’s probably a modification of the parotid salivary gland, with a paired duct. Also, when ejected into the mouth, while there may be oxygen there, it’s enough for only a slow burn (like what Kai did with the thermometers). There’s not enough O2 until it’s blasted into open air, where it’s free to react.
They could probably isolate a sample using a glass syringe from a spinal tap kit. They use glass for change of flow resistance to determine that a needle has accessed the correct depth. Using one, they could draw a sample from the opening with a blunt needle (no need to pierce anything), and obtain a sample with little ignition. They’d want glass, though, because there might be some residual O2 in the air bubbles, and that would produce enough to heat the sample.
We now have mixed signals. A binary pair of chemicals would allow fine control of reaction rate, but a single chemical would probably not burst into flame by itself.
It shows that you know your stuff :p
Well, at least we know Jessie’s adventure is set in summer. It’s night and the Monster Rancher is wearing a sleeveless shirt wiithout looking cold)
His fur might help a little :p
Hmmm..wondering if similar to this:
https://en.wikipedia.org/wiki/Bombardier_beetle
I remember seeing these critters on some show i caught randomly
In relation to the New Years image…I thought Cleo was human, so why does she have a tail???
Edit, just read the Cast page, so found out she’s a Snake hybrid
a real spitfire I say